March 7, 2024
by Baljit Bains, Marketing Communications Specialist, ACD/Labs
ISO10993:18-2020—Simplifying the Chemical Characterization of Medical Device Materials
The presence of extractables and leachables (E&Ls) in medical devices after the manufacturing, packaging, or storage process is unavoidable. E&L analysis is integral to safeguard the safety of medical products and addresses the fundamental questions “What substance(s) may be released from drug packaging or medical devices?” and “Will it cause harm to patients?”
E&L studies require careful design and risk management and must be scientifically justified, considering factors such as device class, application of the device, and contact time. So, it is not surprising that E&L investigations are receiving increased attention from regulatory agencies in recent years.
Regulations govern pre-market approvals, medical device listing, labeling, reporting, electronic signatures, and more. While there are many regulatory guidelines for medical devices, they can be ambiguous—leaving scientists uncertain about whether they have fulfilled all the necessary regulatory requirements.
What are the ISO10993-18:2020 Guidelines?
Depending on the type of medical device (i.e., pre-filled syringes, skin patches, implantable devices, etc.,) it may be necessary to conform to numerous guidelines, rarely does only one guideline apply.
The primary source of regulatory information for manufacturers/suppliers and medical laboratories responsible for combination and medical devices is ISO10993-18:2020. These latest guidelines supersede the previous edition (ISO10993-18:2005), feature updated and expanded flowcharts for the chemical characterization process, and offer more comprehensive definitions to enhance the understanding of analytical testing.
Core elements of ISO10993-18:2020 include determining E&Ls under the conditions of clinical use and the biocompatibility of medical devices.
Chemical Characterization of E&Ls
The ISO10993-18:2020 is a set of standards for characterizing medical devices. The guidelines are broken down into sections, starting with the chemical characterization procedure.
The setup of a chemical characterization study can be summarized in three steps—extraction, detection, and identification and quantification.
Extraction
Extraction aims to test the possible impact and the materials of medical devices. The ISO10993-18:2020 standards guide the selection of the most appropriate extraction conditions.
Selection of the extraction conditions depends on the type of medical device and its specific application. Although it may be necessary to use harsh extraction conditions, there is a fine balance that must be maintained. It is important to not select conditions that are too aggressive and alter the extractable profile.
The types of extraction include:
- Exaggerated Conditions—conditions that are intended to release a greater amount of chemical constituents compared to clinical conditions
- Exhaustive Extraction—multi-step extraction conducted until the amount of material extracted in a single extraction step is less than 10% of that determined in the initial extraction step
- Simulated Use Conditions—extraction using a method that simulates clinical use
Once the extraction type has been selected, other conditions like the extraction solvents, temperature, and time can also be decided.
Detection
The ISO10993-18:2020 guidelines provide recommendations to help select the most appropriate technique for analysis. Depending on the compound, the techniques required to analyze these compounds vary. The figure below shows the technique recommended for a specific category of compound.
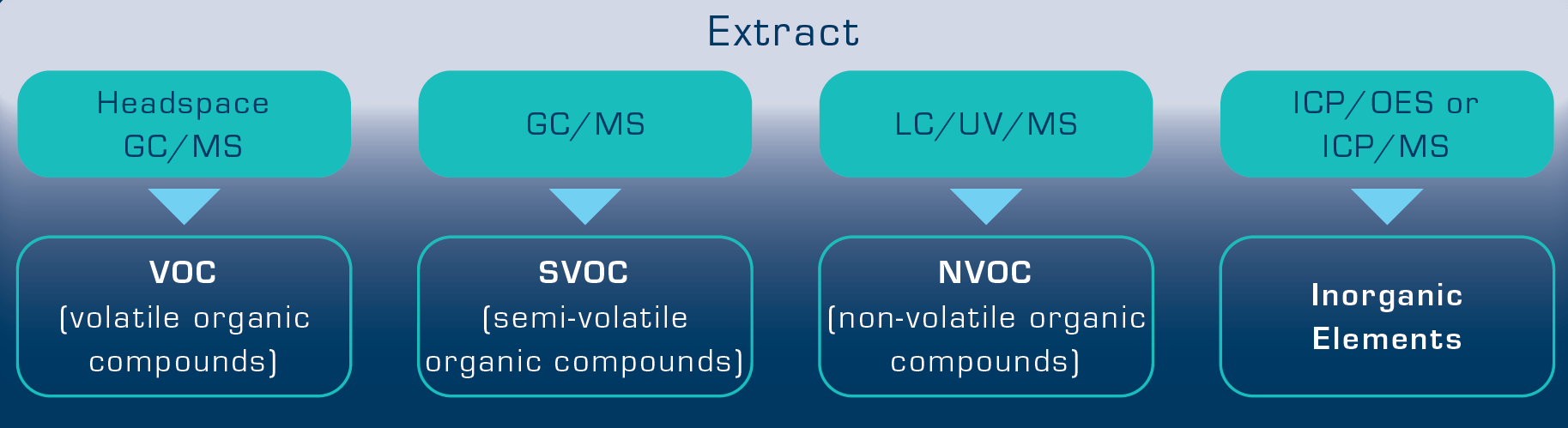
Other factors to consider include:
- Application of the Analytical Technique—how will the technique be applied?
- Screening Approach—screen for every potential compound present in the extract
- Targeted Approach—detect what is known with a low limit of quantification (LOQ) and high accuracy
- Compound Class—to cover all classes of compounds (from volatile to non-volatile) multiple analytical techniques may need to be used
- Ionizable Species—it may be necessary to use high-sensitivity instruments to detect poorly ionized compounds
Considering all the factors mentioned above, the most appropriate analytical technique can be selected and applied, ensuring thorough extraction and detection of all compounds.
A Software Solution for Identification and Quantitation of E&Ls
Compounds detected at or above their toxicological thresholds are targeted for identification and quantitation.
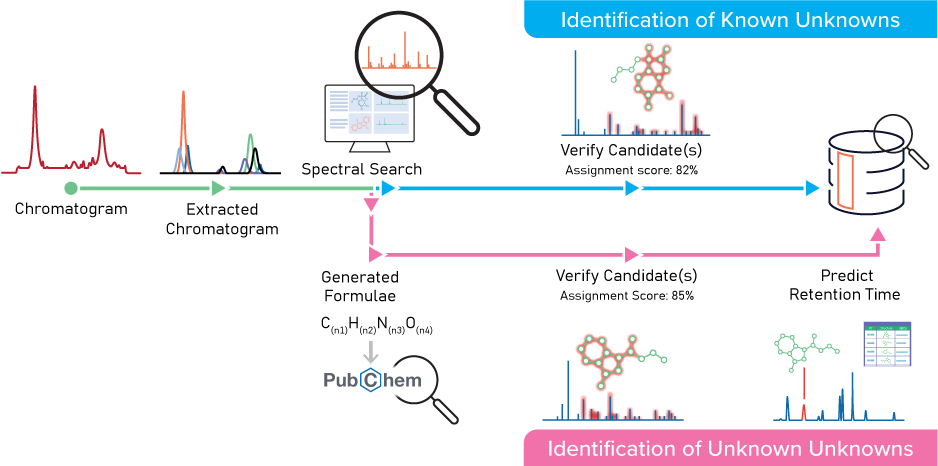
Software like ACD/Labs’ MS Structure ID Suite uses analytical chemistry to help scientists automate and streamline the chemical characterization of E&Ls in medical devices. This software enables users to identify ions present in the spectra, assists with fragment assignment, verifies identifications, and records the results in a centralized database.
The workflow below is an example of how Medtronic uses MS Structure ID Suite to accelerate the chemical characterization of E&Ls. For more details, read the application note.
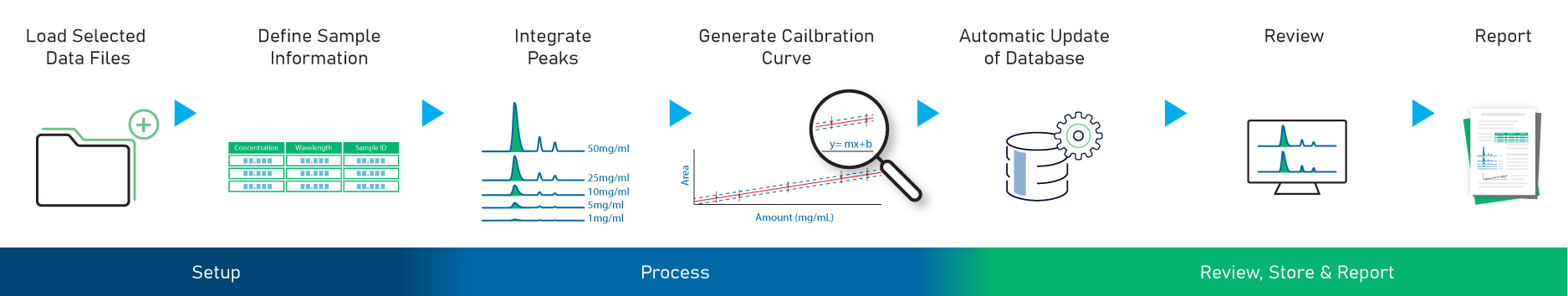
Once the analyte has been identified, the next step is to determine “how much”—the software can be used to generate a calibration curve and quantitate the analytes. The quantitation workflow available in MS Structure ID Suite is shown in the image below. More details about the quantitation workflow can be found here.
MS Structure ID Suite can help accelerate component identification, raise the confidence level of identifications, and improve the accuracy of quantitation—ensuring the safety of your medical device.
Identification Status
ISO 10993-18:2020 requires the identification status of E&Ls to be indicated in chemical characterization reports. However, it is not possible to confirm every single identification with authentic reference sources. To sufficiently identify and report E&Ls it is necessary to establish and use levels of confidence. From high to low confidence, proposed levels are confirmed, confident, tentative, speculative, and unidentified. The higher the confidence level the lower the uncertainty to be used in toxicological assessment.
The highest level of confidence (confirmed identification) can only be used when 3 criteria are met:
- A sufficiently high match factor with a spectrum in a library
- Confirmation of the retention time of the compound on the used instrument
- The use of authentic standards to record the retention time and mass spectrum
When the reported concentration is close to the permitted daily dose of the compound, It is especially important to have confirmed identifications.
Determining the Analytical Evaluation Threshold and Assessing Toxicological Risk
For all products on the market, it is necessary to conduct and submit toxicological risk assessments (TRA) to regulatory authorities. Allowable limits and AET must be considered when conducting a TRA and the process must also conform with other ISO guidelines (ISO10993-17, in particular).
There are detailed recommendations and guidelines within the ISO10993-18:2020 framework to help determine the AET, which acts as the baseline when a chemical poses a toxicological risk. The AET is calculated based on the dosage and the route of administration.
The threshold is set specific to the chemical based on the type of contact and duration of device usage. Chemical species detected at or above this threshold need to be identified, quantitated, and reported for possible toxicological assessment. It is set based on a dose-based threshold (DBT), the lab extraction volume, and the number of devices used for extraction.
Depending on the chemical, the DBT is determined by either:
- Safety Concern Threshold (SCT)—below which a leachable has a dose so low that it presents negligible safety concerns from carcinogenic or non-carcinogenic toxic effects
- Threshold of Toxicological Concern—level of exposure of constituents, below which the risk for human health is not serious
Setting and meeting the AET is not only an important part of the regulatory submission process but also necessary for the safety of medical devices.
Safety Evaluation of Medical Devices
The evaluation of medical devices requires both chemical characterization for a comprehensive assessment of the sensitivity level of chemicals, and toxicology to identify the threshold at which a chemical might pose a risk to patients. The latest ISO10993-18:2020 guidelines provide comprehensive guidance for these processes—enhancing the understanding of analytical testing and improving harmonization with other ISO guidelines.
Find out more about how AstraZeneca manages their E&Ls data, here.
