Method Development Quality Measurement
The time required, personnel cost, and investment (cost of technology required—instruments, software, and consumables) in method development were all direct, straightforward measurements. However, reducing method development time was only one of the goals set by the team at Janssen. In order to measure improvements in quality, the team developed a scale that incorporated key method development quality parameters. This included:
- The number of chromatographic interactions evaluated at once
- The number of variables evaluated within each chromatographic parameter
- The number of design of experiments (DoE) executed
DoE identifies the significant factors that affect the chromatographic separation and optimizes them with respect to method development. The measurement of time, cost, and quality enabled the Janssen team to track progress towards its twin goals and determine a return-on-investment.
In 1997, Rudy and his team at Janssen set out on a 14-year odyssey that combined deployment of new, advanced hardware and software as part of a rational, scientific strategy for method development. According to Rudy, “The two goals we had in our minds were to reduce the method development time and improve the quality of the method development.” The initial step in the scientific strategy was to benchmark the key performance indicators (KPIs) of the present trial-and-error process: development time, personnel cost (for manual operations), investment (cost of technology required—instruments, software, and consumables), and the quality of the results (see sidebar: Method Development Quality Measurement).
“The two goals we had in our minds were to reduce the method development time and improve the quality of the method development.”
– Rudy Sneyers

1997: Screenings Module (LC-UV)
For the first process the Janssen team tried 8 selected columns with different chemistries at 4 pH values (2.5, 4.8, 7, and 9) and screened them simultaneously using liquid chromatography (LC). Final optimization of chromatographic parameters was still adapted in several trial-and-error experiments. As Figure 2 shows, this process reduced development time by almost half and reduced personnel cost by over half. However, the investment increased by 300%, due to the new equipment cost. Quality also improved, but many of the issues with the trial-and-error method remained—the screenings module (LC-UV) process was still complex and time-consuming with no peak tracking between experiments.
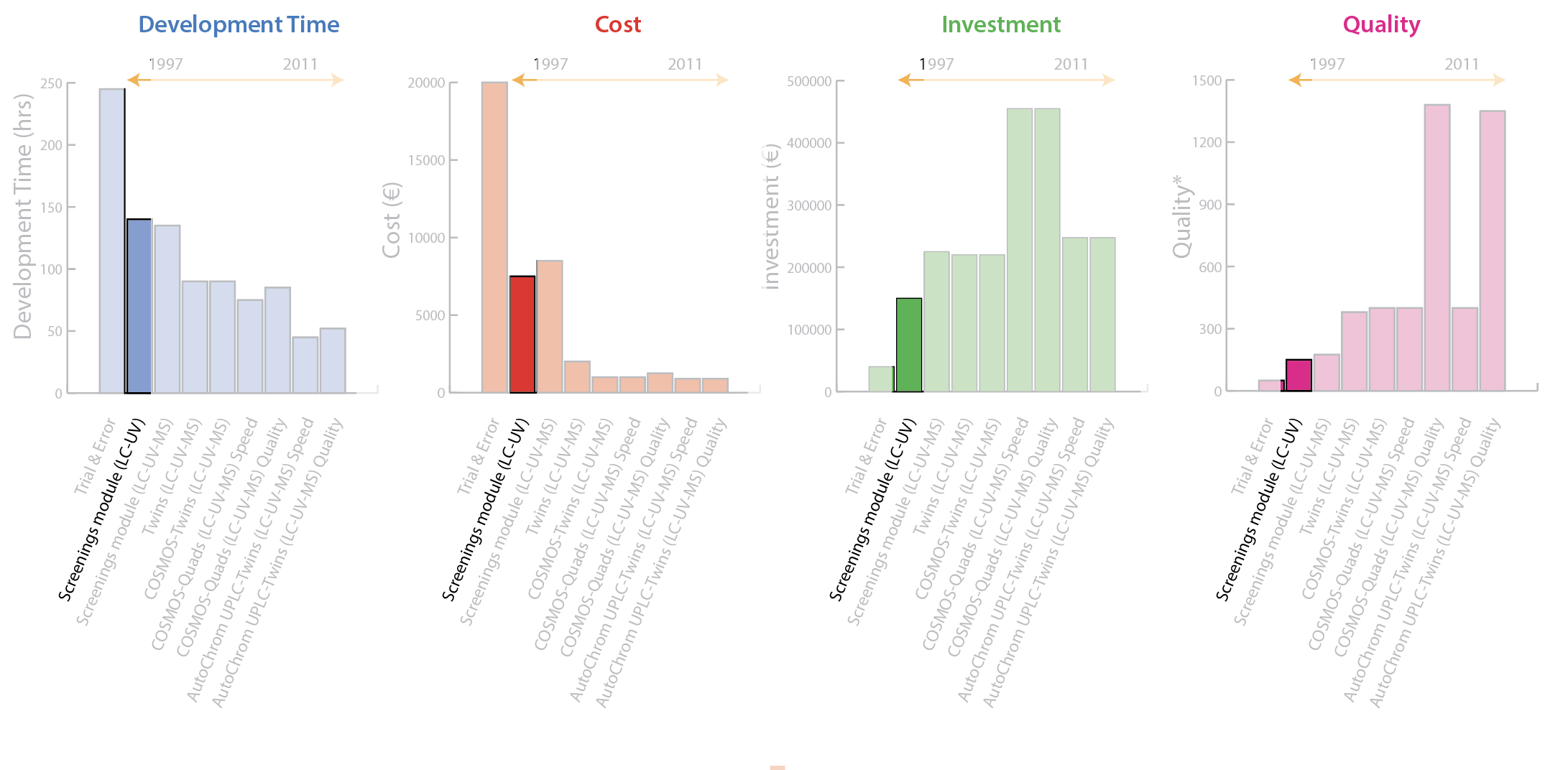
2001: Screenings Module (LC-UV-MS)
The next advancement occurred 4 years later when the outputs of the 4 liquid chromatographs were multiplexed through a mass spectrometer (MS). In this system, 5 columns with different chemistries were selected at 4 different pH values and screened simultaneously. Use of mass detection provided peak tracking between experiments, but final optimization of the chromatographic parameters was still adapted in several trial-and-error experiments. Figure 3 shows a marginal reduction in development time, marginal increase in personnel cost, and a significant increase in investment, due largely to the use of MS data. Quality improved slightly, but manual evaluation of chromatograms remained complex and time-consuming, and the multiplex interface reduced signal-to-noise, thereby producing a loss of sensitivity.
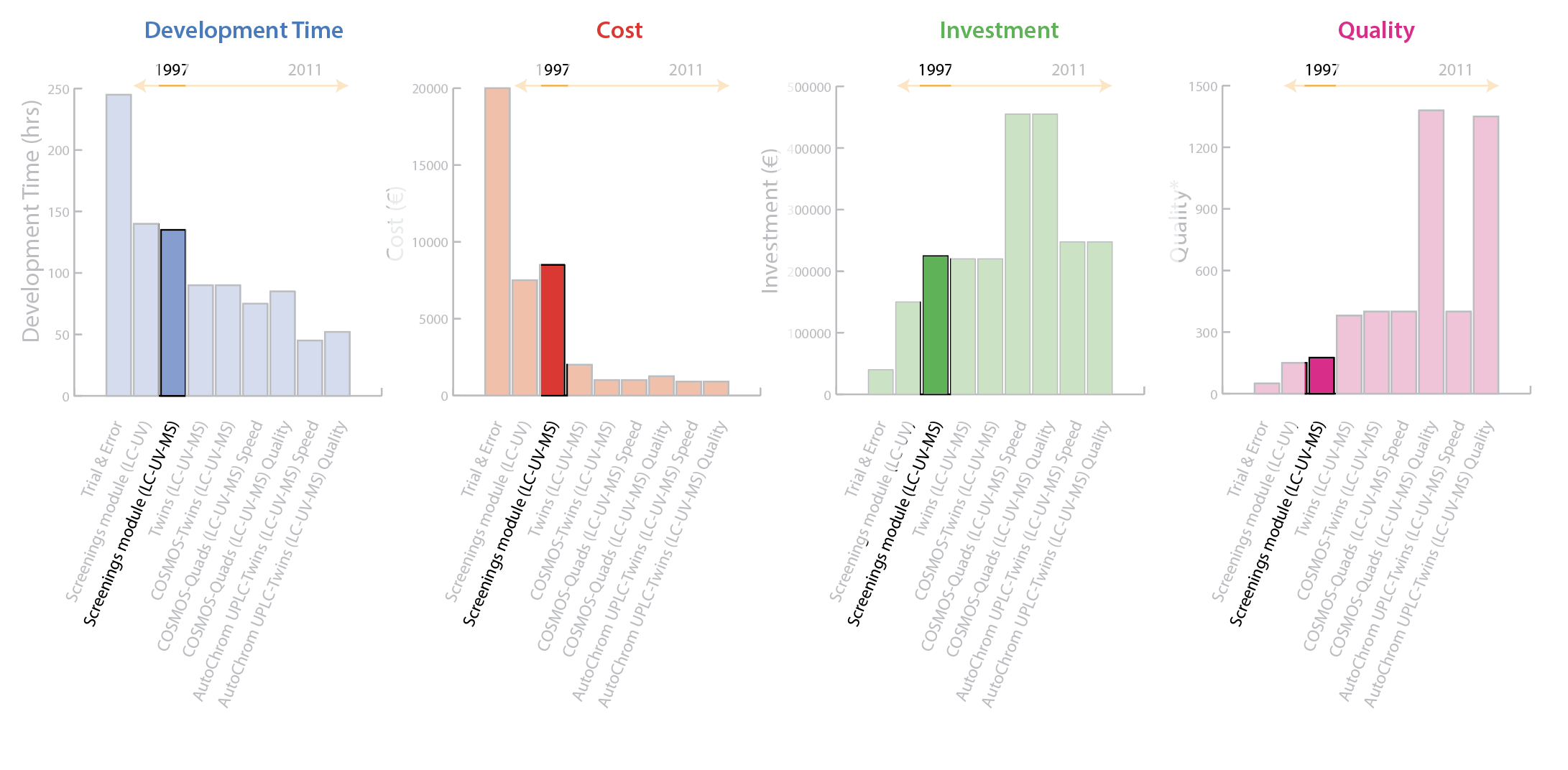
2003: Twins (LC-UV-MS)
The next advancement included a doubling of capacity (twin LC-UV-MS systems) combined with a more scientific approach to chromatographic parameter optimization. Screening a matrix of all chromatographic parameters (full factorial DoE) could take as long as 467 hours, or almost 20 days, for one sub-sample. Because chromatographic columns and pH are two of the most important parameters contributing to selectivity in reversed-phase high-pressure liquid chromatography (HPLC), chromatographic parameters could be optimized in multiple waves based on a scientific approach to DoE. In the first wave, 5 columns are selected at 4 pH values with each column screened simultaneously.
This is the same manner as the prior LC-UV-MS method. When all of the data is collected from this wave, peaks are matched and a resolution map created to identify the optimal pH on each column. In wave 2, seven experiments (derived from the Snyder’s solvent triangle) with different organic modifiers are performed to predict the optimal organic mobile phase composition. In wave 3, four experiments are conducted to predict the optimal gradient slope and column temperature using the selected column from wave 1 with the optimized organic modifier from wave 2. The wave technique reduces the time for optimizing one sub-sample to only 19 hours. Figure 4 shows that this process delivers a significant reduction in development time and personnel cost, with investment remaining about the same as the prior approach. Most impressive is a significant quality improvement. While customized Microsoft® Excel® software is used to build a component table, manual evaluation of chromatograms remained complex and time-consuming.
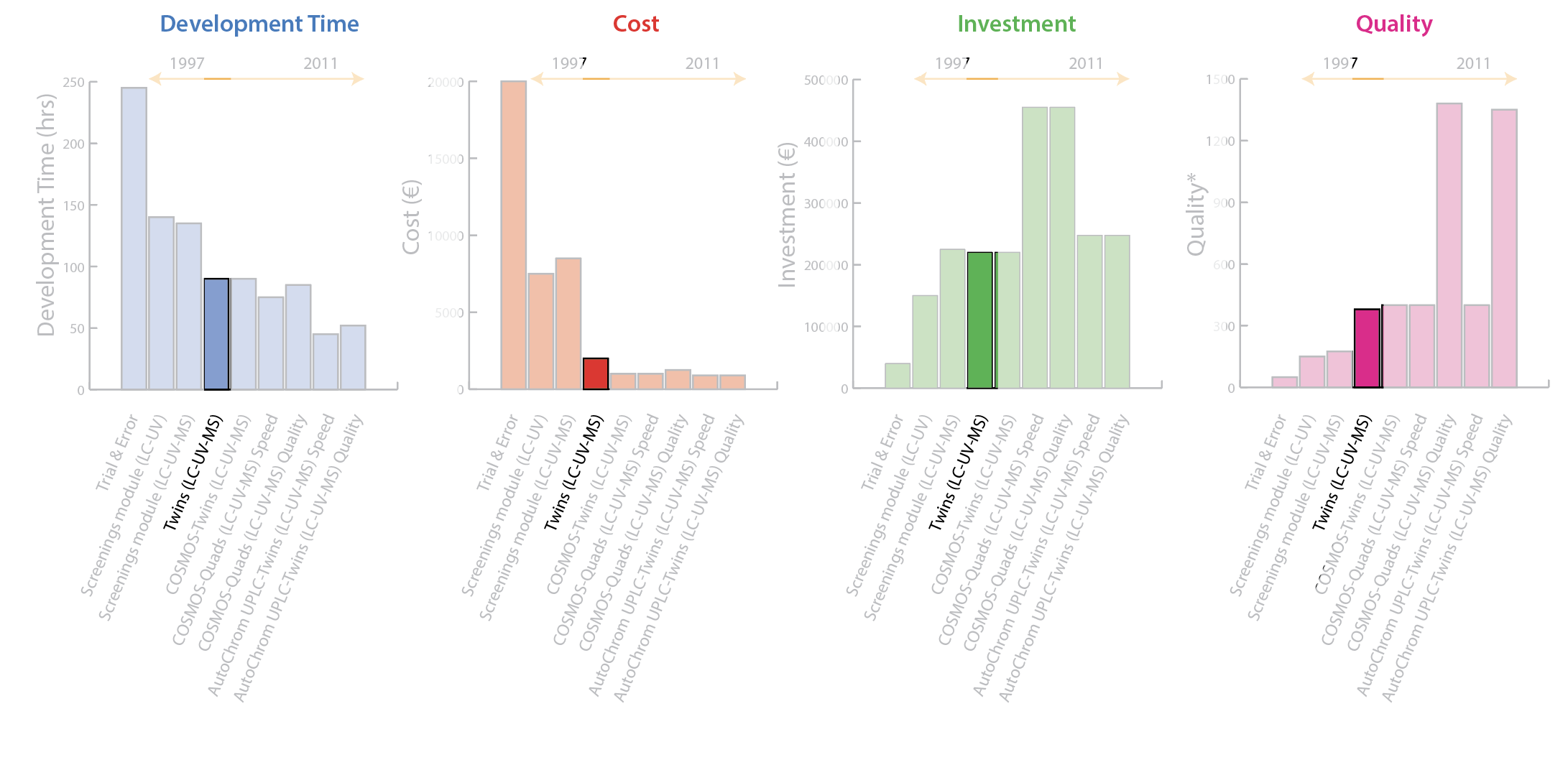
2008: COSMOS–Twins [LC-UV-MS]
The knowledge accumulated from previous processes—especially the disadvantages identified—led to development of the Computer Organized Screening and Method Optimization System (COSMOS) process. This innovative screening and method development process was based on three principles:
- Use of a scientific strategy for DoE informed by prior experience.
- Introduction of a single quadrupole MS along with the Photodiode Array Detector (PDA) detector (LC-MS).
- Specialized commercial software for automated peak tracking and data evaluation.
ACD/AutoChrom software was selected for automated peak tracking and data evaluation. Rigorous method development performed with composite samples and multiple chromatographic detectors results in a tremendous amount of hyphenated data files. ACD/AutoChrom is designed to summarize this data while retaining full links back to the
hyphenated raw data.
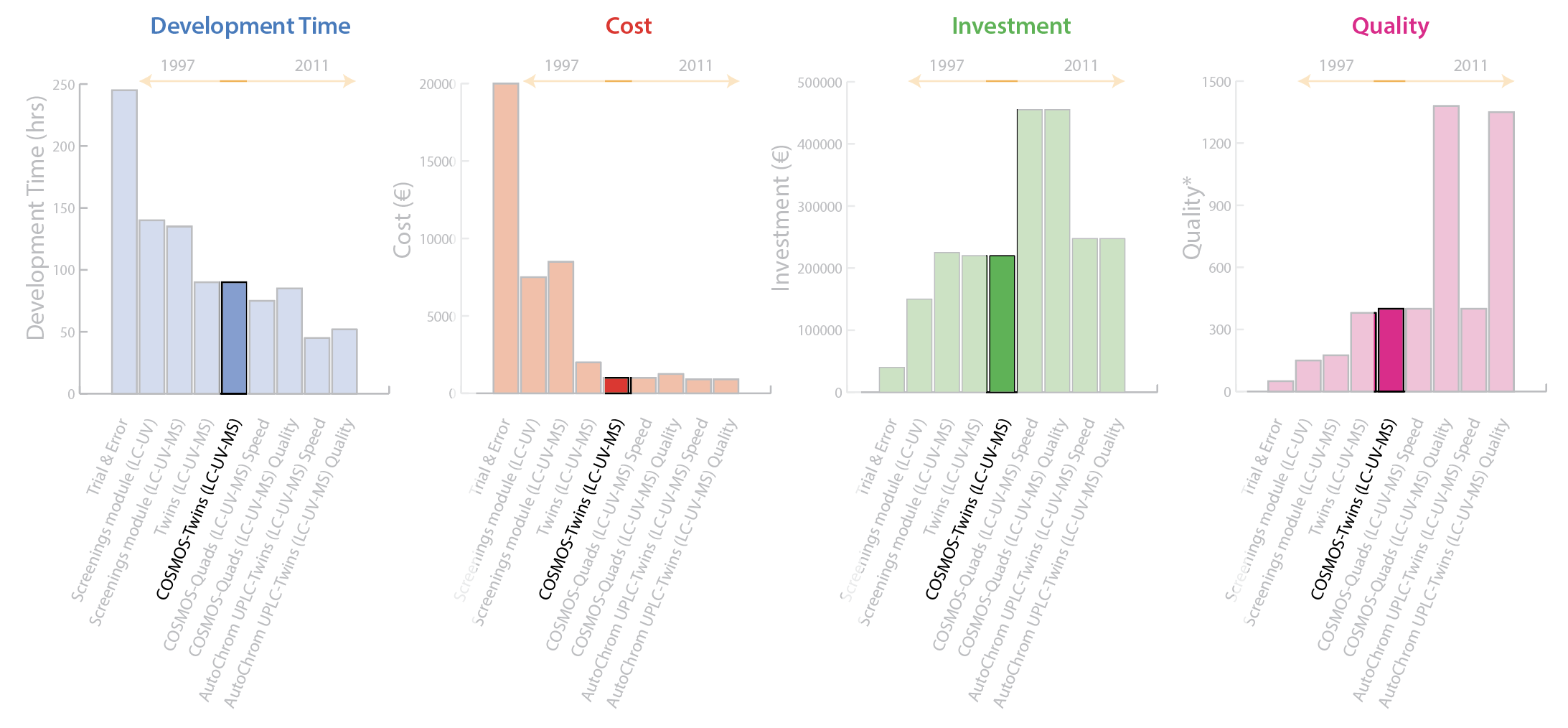
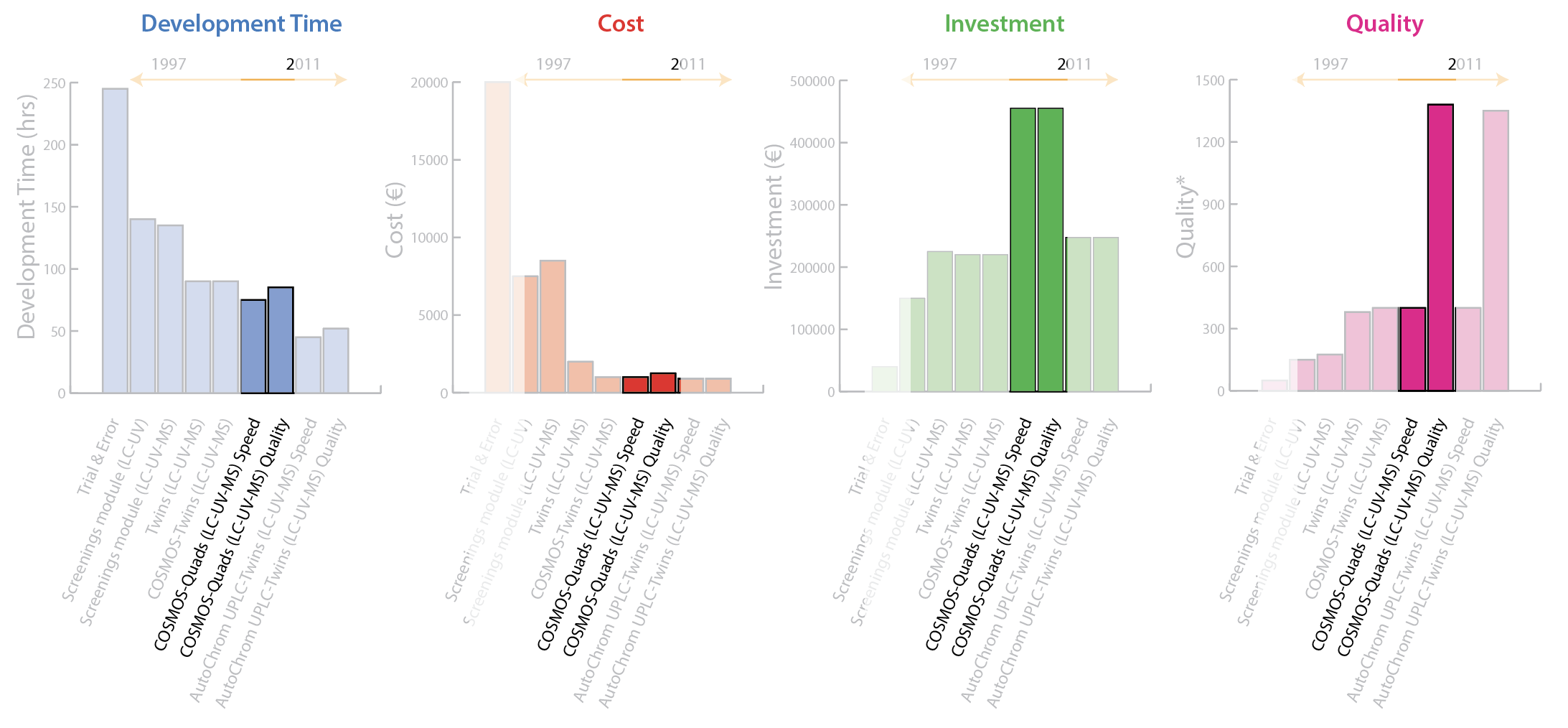
2010: COSMOS–Quads [LC-UV-MS]
By using 4 LC-MS systems it was now possible to speed up method development (speed) or cover more space in the DoE (quality) by combining wave 1 (column screening and pH optimization) and wave 2 (organic modifier optimization) into a single software-enabled operation. Figure 7 shows that Quads [LC-UV-MS] optimized for speed decreases development time slightly with very little change in personnel cost or quality, but with a significant increase in investment. Whereas, Quads [LC-UV-MS] optimized for quality produces very little change in development time or personnel time with the same significant increase in investment, but it delivers a dramatic improvement in quality.
2011: AutoChrom UPLC–Twins [LC-UV-MS]
Combining the high resolution and speed of Ultra Performance Liquid Chromatography (UPLC) with advanced MS detectors and software tools significantly improves the efficiency of method development for less investment than Quads [LC-UV-MS]. As Figure 8 shows, AutoChrom UPLC–Twins [LC-UV-MS] delivers the fastest development time and the lowest personnel cost. While the quality result for AutoChrom UPLC–Twins [LC-UV-MS] is about the same as Quads [LC-UV-MS]—whether optimized for speed or quality—the investment is much less.

Over a 14-year period, the small molecules method development team in the analytical group at Janssen Pharmaceuticals led by Rudy Sneyers pursued a methodical process to improve both time and quality. As Rudy noted…
“I told my colleagues it has to be possible to reach both of these goals, and, indeed, we reached them.”
– Rudy Sneyers
A comparison of the key performance indicators for AutoChrom UPLC–Twins [LC-UV-MS] with those of the trial-and-error starting point substantiate this claim: Janssen achieved an 80% reduction in method development time, and a 25-fold improvement in quality. Furthermore, the investment for AutoChrom UPLC–Twins [LC-UV-MS] is only slightly more than the savings in personnel cost, due in large part to the automation of manual operations, especially the raw-data evaluations of multiple chromatographs.
The odyssey also led the Janssen team to several important insights:
- The use of mass spectrometry with dedicated evaluation and modeling software is an innovative way to perform method development based on scientific principles.
- The Computer Organized Screening and Method Optimization System (COSMOS) significantly improves the quality of the methods, reduces development time and costs, and reduces loss of interpretation information and expensive retesting of samples.
- With the hyphenated data from modern instruments and the available computer capacity, ACD/AutoChrom is a tool for organizing, visualizing, and tracking the data, retaining full links back to the original raw data. According to Rudy, “AutoChrom and the LC simulator improved the quality of the methods because it is based on the design of the experiments or quality by design. The software improved both the quality of the method and the method development time.”
- AutoChrom UPLC–Twins [LC-UV-MS] is a quality-by-design approach in chromatographic method development that will recover the investment costs within a very short time and deliver positive returns thereafter.
“AutoChrom and the LC simulator improved the quality of the methods because it is based on the design of the experiments or quality by design. The software improved both the quality of the method and the method development time.”
– Rudy Sneyers
Download Case Study
Download Now
About Advanced Chemistry Development, Inc. (ACD/Labs)
ACD/Labs is a cheminformatics company that develops and commercializes solutions in support of R&D. Our solutions are used globally in industries that work with small molecules including pharma/biotech, chemicals, consumer goods, agrochemicals, petrochemicals, academic institutions, and government organizations. We provide integration with existing informatics systems and undertake custom projects including enterprise-level automation. 2014 marks our twentieth anniversary of helping organizations accelerate R&D and leverage corporate intelligence. ACD/Labs also provide worldwide sales and support with offices in N. America, Europe, and Asia.
For more information, please visit www.acdlabs.com.
About Janssen Pharmaceuticals, Inc.
As a member of the Janssen Pharmaceutical Companies of Johnson & Johnson, Janssen Pharmaceuticals, Inc. is dedicated to addressing and resolving the major unmet medical needs of our time. Driven by our commitment to patients, healthcare professionals, and caregivers, we strive to develop sustainable and integrated healthcare solutions by working in partnership with all stakeholders on the basis of trust and transparency. Our daily work is guided by meeting goals of excellence in quality, innovation, safety, and efficacy in order to advance patient care.
For more information on Janssen Pharmaceuticals, Inc., visit us at www.janssenpharmaceuticalsinc.com.
