Pfizer’s Approach with Method Development Software
Chromatographic methods are crucial for separating and analyzing complex mixtures of compounds. Method development is a laborious and time-consuming process where chromatographers must maintain a fine balance between achieving the best resolution between peaks (especially the critical pairs) and doing so with the shortest runtime. Increased collaboration across facilities and partners (i.e., CxOs) for all analytical laboratories means that labs frequently transfer methods.
Method transfer involves adapting a method to a different chromatography system—this could be different equipment within the same lab, or equipment within a different lab (i.e., CxO). Method transfer can occur at several stages during research and development, and variability in instrument-to-instrument performance is common.
To ensure successful method transfer between labs, the method must perform consistently, and the results should be replicable. This requires a thorough understanding of the cause and effect of varying parameters. Typically, analytical labs use manual processes to determine the cause of differences in chromatographic separations. However, these processes are time-consuming and require expert knowledge of the method parameters, software, and processes. To overcome these challenges, a workflow using ACD/Method Selection Suite software has been developed in collaboration with the Separation Science SME team at Pfizer.
The Evolution of Method Transfer at Pfizer
Before transferring methods between internal groups and partner laboratories, project teams must assess analytical methods against variations in method parameters and understand the impact on chromatographic separation.
Traditionally, this involved a combination of digital simulation and practical experimentation to adjust various parameters and observe the outcome. This was a laborious, time-intensive process, taking up to several days of investigation and experimentation with frequent back-and-forth between partners.
To address this challenge, Pfizer’s method development team developed a workflow using Method Selection Suite software. The Separation SME team manually adjusted parameters by a small, deliberate variation from the center point, to create simulations of methods for the different combinations of parameters using the LC Simulator tool. This approach generated a series of chromatograms to showcase possible variations (i.e., resolution, overlapping peaks), all within the separation requirements, and provide insight into the method conditions to relay to the receiving lab. Although effective, this process was still laborious and time-consuming (taking up to a full day) and required expert knowledge of the software and processes.
The simulated methods workflow in LC Simulator, however, laid the foundation for a new process to determine the method that most closely matches the original method conditions and parameters. ACD/Labs and the Separation SME team collaborated to create a feature within Method Selection Suite to vary method parameters. This vary method parameters tool replaces the manual effort of the separation scientist to run adjusted parameters through the software with a few button clicks. Methods are automatically generated for all possible combinations of parameter variations—enabling seamless method transfer without impacting method performance.
Efficient, Automated Method Transfer Using Method Development Software
Slight deviations from method conditions can significantly impact chromatographic separations. Methods are developed and reported to partners to ensure that small variations in conditions upon transfer do not impact critical attributes like resolution.
Automatically Generate Methods for All Combination Variations
The vary method parameters tool allows the parameters of a developed method to be varied by specified amounts and automatically generates methods for each possible combination of variations. Parameters can be adjusted, and methods simulated either one at a time or simultaneously. The resulting chromatograms can highlight the highest variation expected under given parameters and conditions and warn the project team of the risks.

Figure 1. The vary method parameters window provides information on the number of methods generated based on the input variations.
The created methods with chromatographic information, metadata, and method details can be visualized within the software (as separate documents or combined as a series) and shared with partners.
Ensure Quality and Robustness
Variations in parameters can influence chromatographic behavior to different extents. Using the feature to vary method parameters allows the chromatographers to predict chromatographic behavior within specified bounds—ensuring the replication of methods in different labs while maintaining quality and control.
The success of method transfer depends on the intended application of the method and which parameters (edge of failure of the resolution map, critical parameters, or impactful instrument parameters such as pump type or dwell volume) are of interest. To ensure adequate control of methods, it is essential to identify critical parameters that significantly impact the chromatographic output (i.e., peak shift, resolution selectivity alteration, etc.). Using the vary method parameters tool, chromatographers understand the impact method parameters have on the resulting chromatogram. This helps them determine whether anomalies between transferred methods result from the variation of one or a combination of certain method critical parameters, allowing prompt implementation of corrective actions, or factors beyond their scope, thus preventing unnecessary experimentation. Understanding how parameters influence the method’s quality is crucial for saving time and preventing method failure during transfer.
Deeper Insight into Methods with Visualization Tools
Method Selection Suite software provides deeper insight into the method development process through visualization of chromatograms and resolution maps, helping separation scientists to quickly observe differences between experimental and predicted methods. Overlay of chromatograms helps scientists to identify variations and pinpoint the specific parameter or combination of parameters responsible. Armed with this knowledge, scientists can address specific problems and/or implement appropriate control measures.

Figure 2. Visualization of experimental and predicted chromatograms.
These visualization tools include resolution maps with a “center point” which helps identify optimal separation conditions and ensure maximal method robustness, where the resolution is least affected by parameter variations.
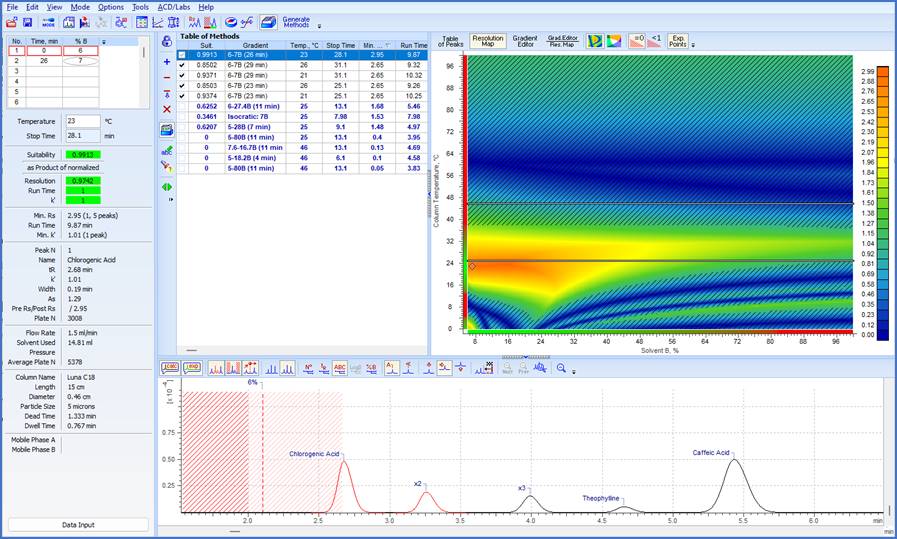
Figure 3. An example of a resolution map with the center point for the highlighted method displayed. The Table of Methods and chromatogram for the highlighted method are also shown.
Integrated Data Management for Easy Data Accessibility and Collaboration
Differences in hardware (columns, detectors, chromatographic systems), especially between different instrument vendors, cause variability in instrument-to-instrument method performance. Method Selection Suite software integrates different systems, regardless of vendor or location, enabling consistency of methods across various setups.
At Pfizer, different types of analytical data from many instruments are stored within an Enterprise database on the cloud. The data, in the Spectrus file format, can be processed and analyzed in the software. Processed chromatograms from Empower, methods (original and varied parameters), metadata, structures, retention time, etc., are automatically added to the easily searchable and accessible database. Following this, customized reports can be generated to communicate the information to the project team and receiving analyst. Clean and clear reporting facilitates more effective communication and collaboration between internal colleagues and partner organizations, streamlining the sharing of knowledge, experiences, and method challenges.
Maintain Control, Consistency, and Reproducibility of Methods
Successful method transfer requires the method to be robust and reproducible and to preserve its analytical performance metrics such as accuracy and quality, across diverse lab environments and over time. The vary method parameters tool within Method Selection Suite software helps method developers identify the most robust starting method and develop a deeper understanding of critical method parameters. Teams can leverage this knowledge when migrating methods to partner labs, to account for and control potential variations in parameters.
The vary method parameters tool helps users confidently approach chromatographic method transfer—saving time and resources, enhancing their understanding of chromatographic quality for finer control over methods, and streamlining communication between the developer, project team, and receiving partners.

